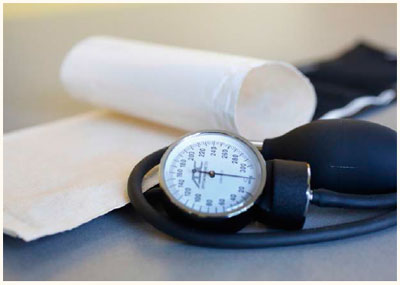
Biovation has launched the BioArmour Blood Pressure Cuff Shield (BPCS) to mitigate the spread of infectious pathogens from blood pressure cuffs. This first-in-class medical product offers a multi-use barrier for prevention of hospital-acquired infections (HAIs). The BPCS has been designed, tested and validated over a two-year cycle. BioArmour is the first in a suite of infection and pathogen control products from Biovation.
The easy-to-use BioArmour BPCS is a disposable antimicrobial hygienic barrier that attaches to the blood pressure cuff to prevent direct contact of the cuff with the patient’s skin. The latex-free shield material is composed of a sustainable biopolymer impregnated with antimicrobial and antifungal agents to mitigate the propagation of a wide spectrum of pathogens in controlled release fashion. Contaminant pathogens – including MRSA, VRE, c. difficile and others – are mitigated by the blood pressure cuff shield, allowing for multi-patient use over a 24-hour period.
 In lab testing, the BioArmour BPCS has been shown to kill up to 99.99 percent of tested microorganisms. The BPCS is manufactured with green technology and a sustainable biopolymer, with an industry-leading 74 percent biocontent. Its slim profile does not adversely impact accuracy of the blood pressure measurement data. And, the BPCS provides another tool for risk mitigation for hospitals and health care facilities with proactive measures for infectious disease reduction and control. This cost-effective system requires no staff maintenance or disinfection between patients and is targeted for in-patient, physician offices, hospice and nursing home facilities and emergency medical services.
In lab testing, the BioArmour BPCS has been shown to kill up to 99.99 percent of tested microorganisms. The BPCS is manufactured with green technology and a sustainable biopolymer, with an industry-leading 74 percent biocontent. Its slim profile does not adversely impact accuracy of the blood pressure measurement data. And, the BPCS provides another tool for risk mitigation for hospitals and health care facilities with proactive measures for infectious disease reduction and control. This cost-effective system requires no staff maintenance or disinfection between patients and is targeted for in-patient, physician offices, hospice and nursing home facilities and emergency medical services.
The BioArmour BPCS has been registered a CE Class I medical device marked for distribution in the EU. As a medical product, it is available for sale in Canada and all other non-U.S. countries. It is currently undergoing testing for FDA approval, which is anticipated to be received in late summer 2015.