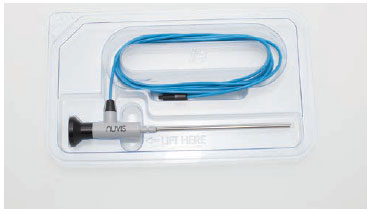
Integrated Endoscopy Inc. has launched the nuvis single-use arthroscope at a time of heightened awareness about serious patient safety issues connected with endoscopic procedures, including a number of recent high-profile infection outbreaks caused by bacteria build up in difficult-to-clean endoscopes.
“We’re excited to introduce the nuvis single-use arthroscope to orthopedic surgeons,” said George Wright, president and CEO of Integrated Endoscopy. “Our discussions with orthopedic surgeons across the country underscore the importance of exceptional optical quality in a disposable endoscope. The nuvis arthroscope is the first single-use rigid endoscope based on 21st century optical technology. Its excellent optics and improved safety provide first-time quality for every procedure, benefiting surgeons and patients alike.”
 The nuvis single-use arthroscope features 12 molded glass lenses. These high-quality lenses are made with the same technology and low-temperature glass used to mass produce high-definition, low-cost optics for smartphone cameras. The nuvis arthroscope also features patented light-emitting diode (LED) technology that provides improved white light at significantly lower temperatures than the fiber optic illumination used in conventional endoscopes.
The nuvis single-use arthroscope features 12 molded glass lenses. These high-quality lenses are made with the same technology and low-temperature glass used to mass produce high-definition, low-cost optics for smartphone cameras. The nuvis arthroscope also features patented light-emitting diode (LED) technology that provides improved white light at significantly lower temperatures than the fiber optic illumination used in conventional endoscopes.
The nuvis single-use arthroscope received 510(k) clearance from the U.S. Food and Drug Administration in July 2014. Integrated Endoscopy is currently gearing up production of the nuvis arthroscope, and it will be available nationwide during the third quarter of 2015. •