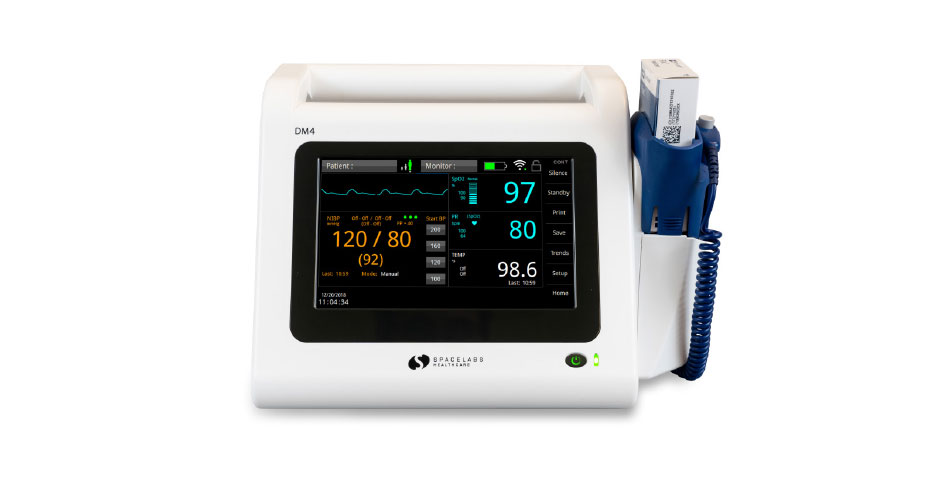
 Spacelabs Healthcare’s DM4 dual-mode monitor is the newest member of the company’s patient monitoring line. The DM4 is designed for use in any patient care environment where basic vital signs monitoring is needed, and it is expected to enable caregivers to adapt to changes in patient condition without having to locate additional equipment. In spot-check mode, the DM4 can wirelessly transmit individual patient records to an electronic medical record system (EMR), or it can be used to efficiently collect and download multiple patients’ records in batch mode from a central location. If more vigilance is required, the DM4 can be quickly switched to continuous monitoring mode with alarm notification support.
Spacelabs Healthcare’s DM4 dual-mode monitor is the newest member of the company’s patient monitoring line. The DM4 is designed for use in any patient care environment where basic vital signs monitoring is needed, and it is expected to enable caregivers to adapt to changes in patient condition without having to locate additional equipment. In spot-check mode, the DM4 can wirelessly transmit individual patient records to an electronic medical record system (EMR), or it can be used to efficiently collect and download multiple patients’ records in batch mode from a central location. If more vigilance is required, the DM4 can be quickly switched to continuous monitoring mode with alarm notification support.








