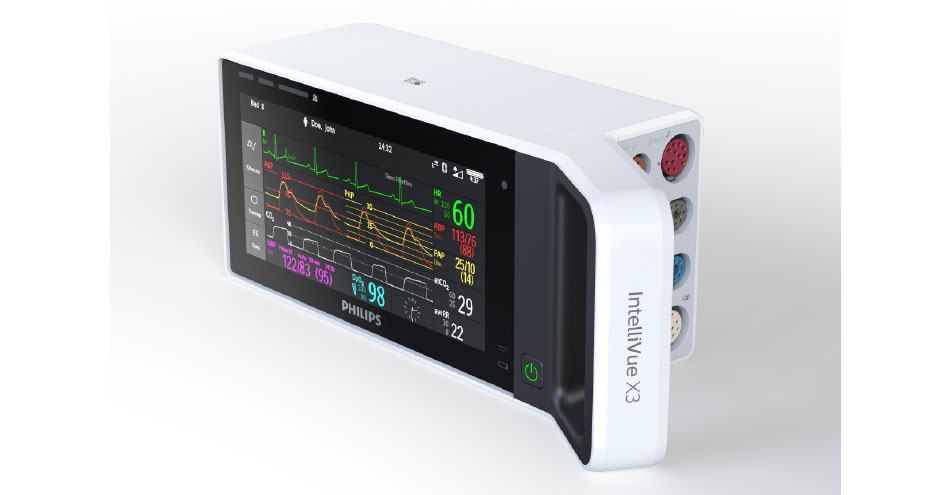
Philips IntelliVue X3 is a highly portable, dual-purpose monitor with intuitive smartphone-style operation that helps streamline workflow and boost efficiency by reducing the number of steps to prepare patients for transport. With this monitor, there is no need for clinicians to change patient cables during transport or at bedside, allowing them to spend less time dealing with equipment and more time caring for the patient across all levels of acuity. In addition to integrating seamlessly into the Philips Patient Monitoring solution, data from IntelliVue X3 is also integrated into mobile applications, the hospital network, and interfaces that connect the system to other medical devices and the hospital’s EMR system for virtually gap-free patient information from admission to discharge – even during transport.








