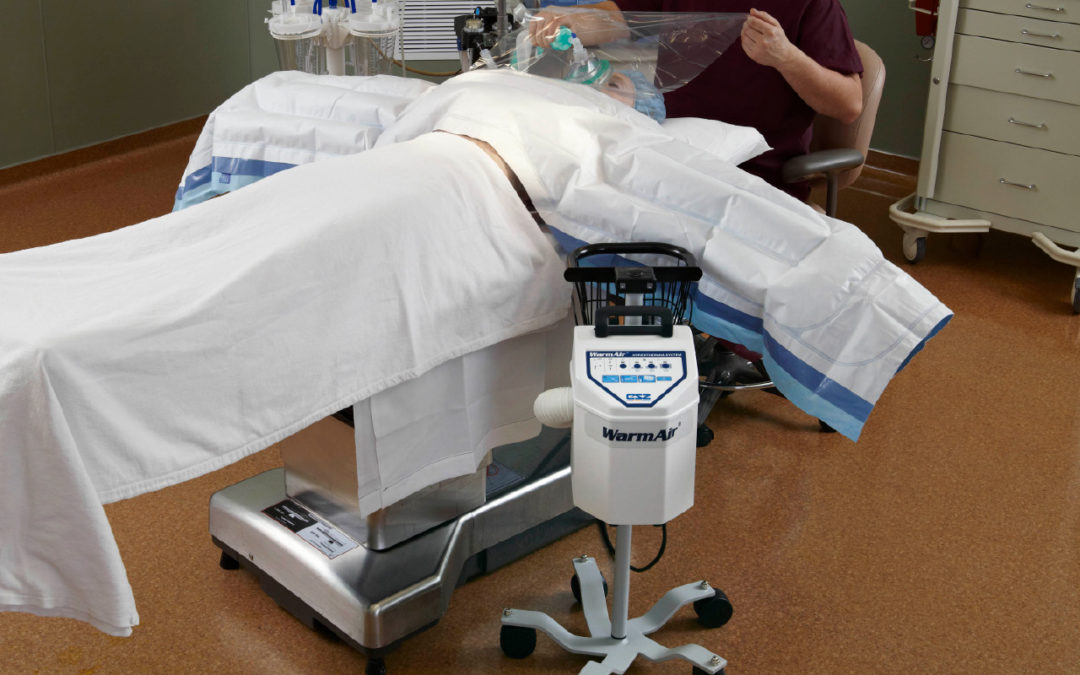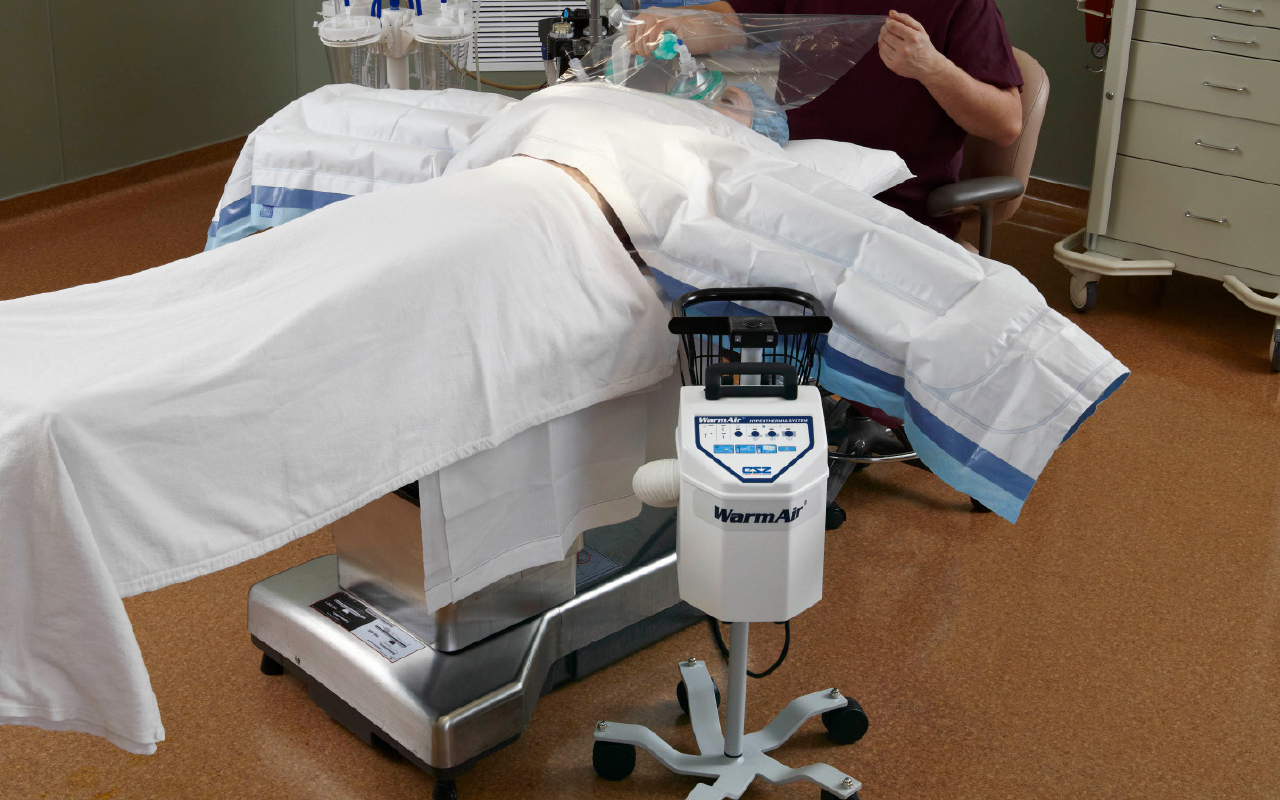
Gentherm’s WarmAir patient warming system is a compact and quiet unit that delivers gently moving warm air using a low velocity blower. All air is filtered through the WarmAir unit and the FilteredFlo blankets. With built-in safety monitoring systems, caregivers will be alerted if the temperatures exceed or fall below temperature settings, providing further protection to a patient. It helps keep patients comfortable before and after surgery and maintains body temperature during surgery. Gentherm uses Filtered Air Warming which is a unique patented design of a FilteredFlo blanket that permits usage of a lower velocity blower to supply gently moving, filtered air.