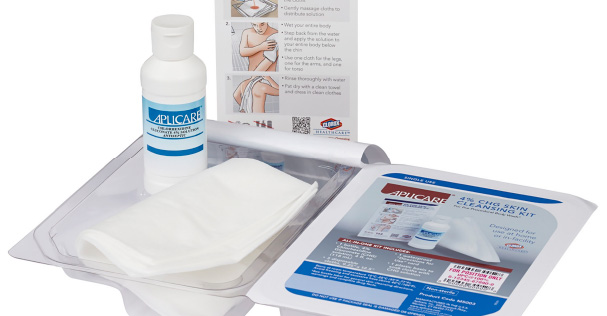
The Clorox Healthcare™ 4% CHG Skin Cleansing Kit is an easy-to-use kit for pre-procedural skin cleansing designed to help improve patient compliance. Two packs, attached via a perforated seal, contain the essentials for two chlorhexidine gluconate (CHG) cleanses including a 4 ounce bottle of CHG, 3 cloths and a bilingual, waterproof instruction card. To help health care professionals deliver better patient outcomes, Clorox Healthcare has also issued a white paper that details the clinically proven benefits of multiple CHG skin applications as part of a strategy to reduce SSI risk. To download a copy of the white paper, visit www.CloroxHealthcare.com/CHGSkinPrep. •