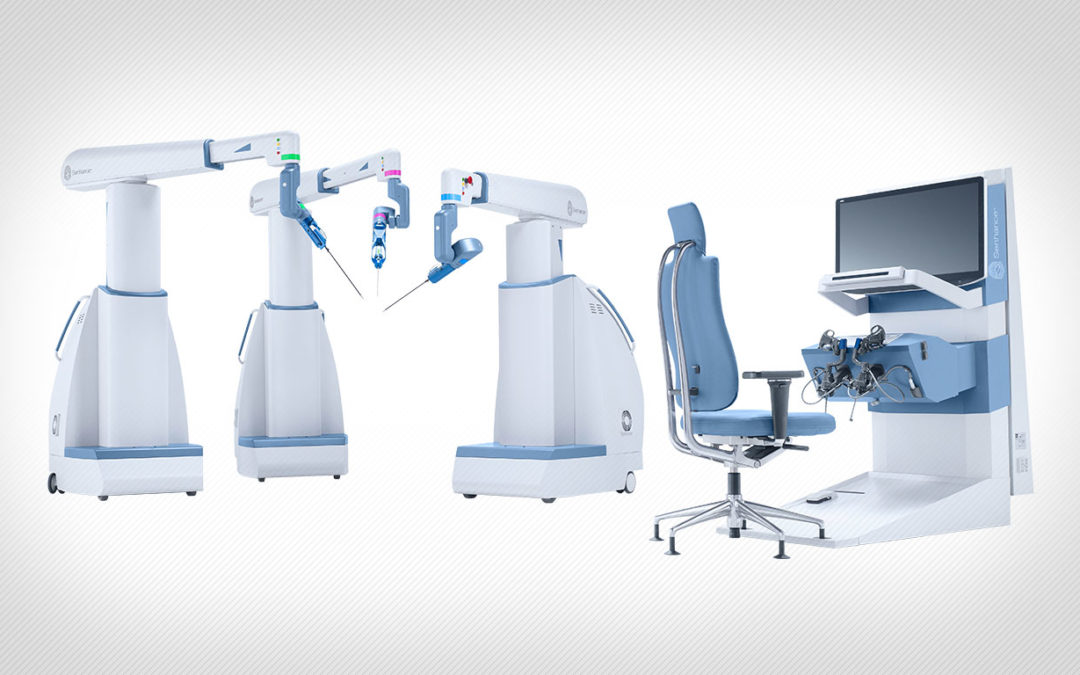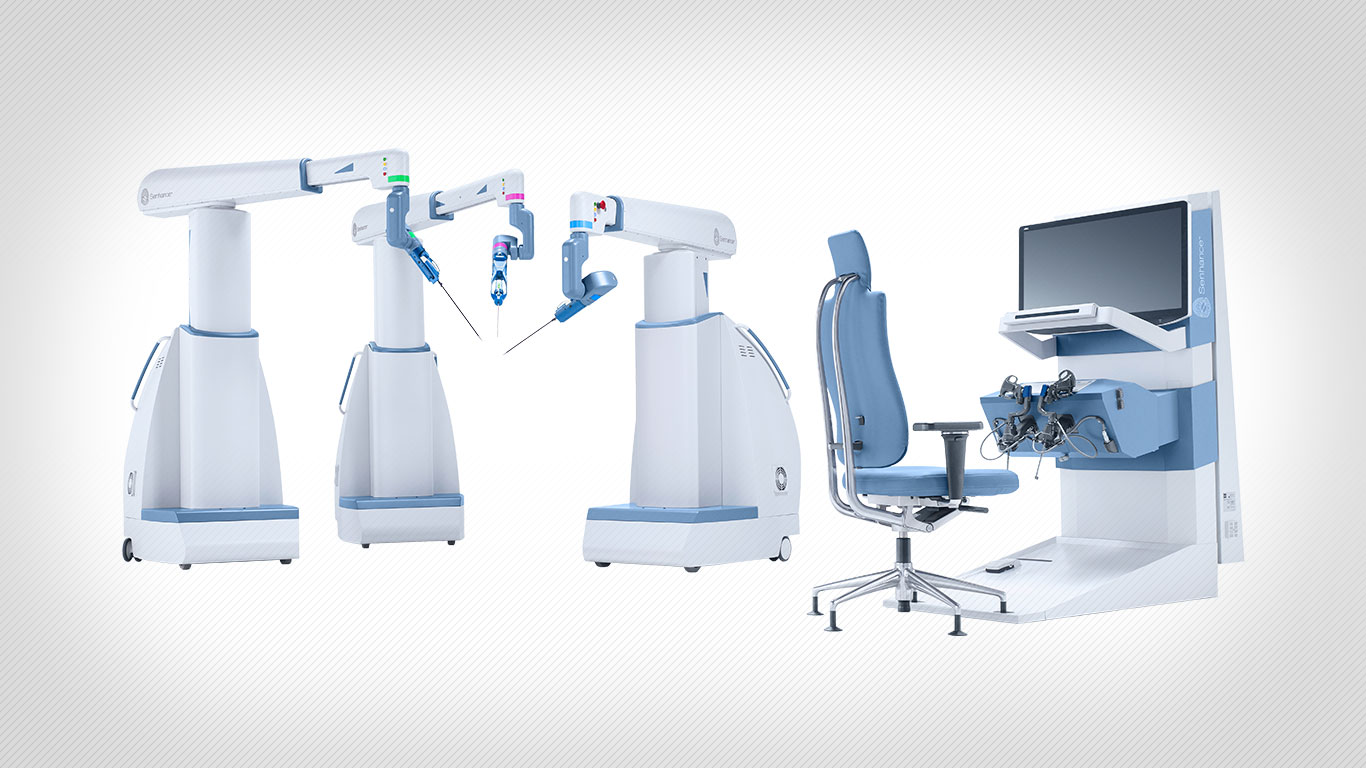
Asensus Surgical Inc. (formerly TransEnterix Inc.) has received an additional FDA clearance for the Senhance Surgical System which allows for indication expansion in general surgery in the United States.
A news release states that Asensus Surgical’s technology platform, Senhance Surgical System, is the first of its kind digital laparoscopic platform that leverages augmented intelligence to provide unmatched performance and patient outcomes through machine learning. Senhance goes beyond the typical surgical robotic systems, providing surgical assurance through haptic feedback, eye-tracking camera control, and 3D visualization, and is the first platform to offer 3 mm instruments (the smallest instrument available in the world on a robotic surgical platform).
“The expansion into general surgery for the Senhance Surgical System is a major milestone for the growth and clinical applicability of our technology,” said Anthony Fernando, Asensus Surgical president and CEO. “General surgery is, by far, the largest area of manual laparoscopy which can benefit from the precision and insight of performance-guided surgery. The indication expansion allows Senhance to be used in many high-value, complex reconstructive surgeries such as those used to treat reflux and obesity. Including previous indications granted, the Senhance Surgical System can now be utilized in over 2.7 million general surgical procedures performed in the U.S. annually.”
“Many of the procedures we perform in general surgery require complex reconstruction throughout a wide surgical field,” said Dr. Sabino Zani, assistant professor of surgery at Duke University and an investigator in the clinical studies submitted for indication expansion. “Senhance can now be seen as a widely applicable tool for general surgeons across the broad range of procedures that may be performed from deep in the pelvis to the upper abdomen.”
The Senhance Surgical System is intended to assist in the accurate control of laparoscopic instruments for visualization and endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrocautery, suturing, mobilization and retraction. The Senhance Surgical System is intended for use in general laparoscopic surgical procedures and laparoscopic gynecological surgery. The system is indicated for adult use. It is intended for use by trained physicians in an operating room environment in accordance with the instructions for use.
For more information, visit www.senhance.com.