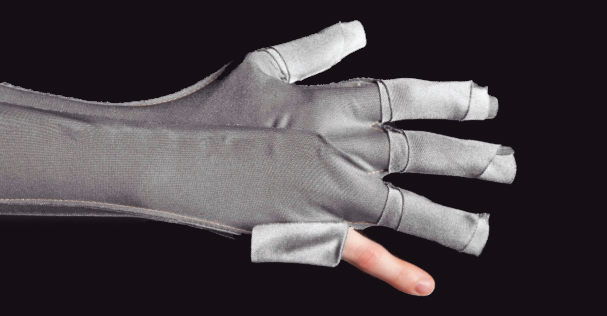
Ansell is changing the dressing treatment paradigm with the launch of GAMMEX™ Burn Treatment Solutions. The latest launch features novel, fully customizable solutions for the hard to dress areas in burn and wound care, including the first fully customizable silver antimicrobial barrier glove, featuring Velcro™ closures for adjustable compression and localized wound evaluation, and an outer dressing that offers superb fluid management 2x that of gauze. The line is part of a team therapy approach to burn and wound care, offering efficient, reliable and consistent application across providers. The launch includes four products compatible across treatments: GAMMEX™ Silver Barrier Glove, Silver Finger Dressing, Outer Dressing and Outer Finger Dressing. For more details, visit www.ansell.com/gammexburn.