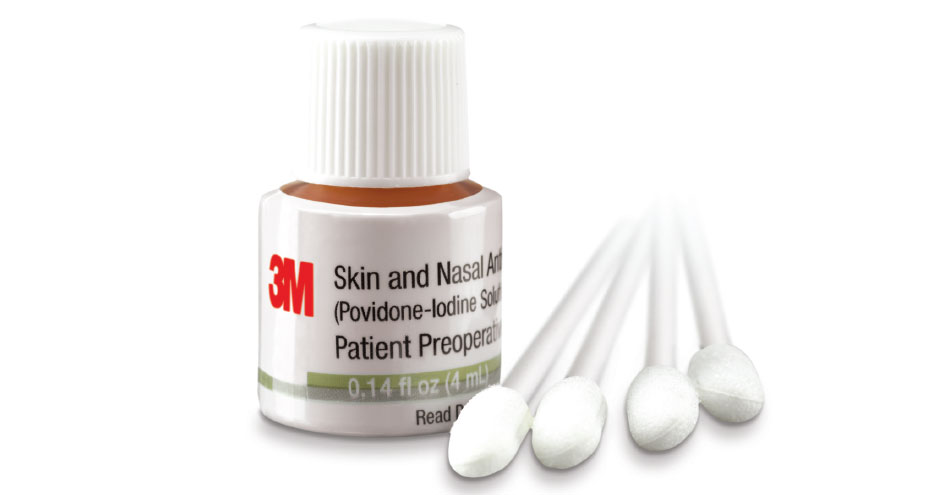
Providers can take control of preoperative nasal decolonization with 3M Skin and Nasal Antiseptic (Povidone-Iodine Solution 5% w/w [0.5% available iodine] USP) Patient Preoperative Skin Preparation. This simple, one-time application reduces nasal bacteria, including S.aureus, by 99.5% in just one hour and maintains this reduction for at least 12 hours.[1]
1. 3M Study-05-011100.