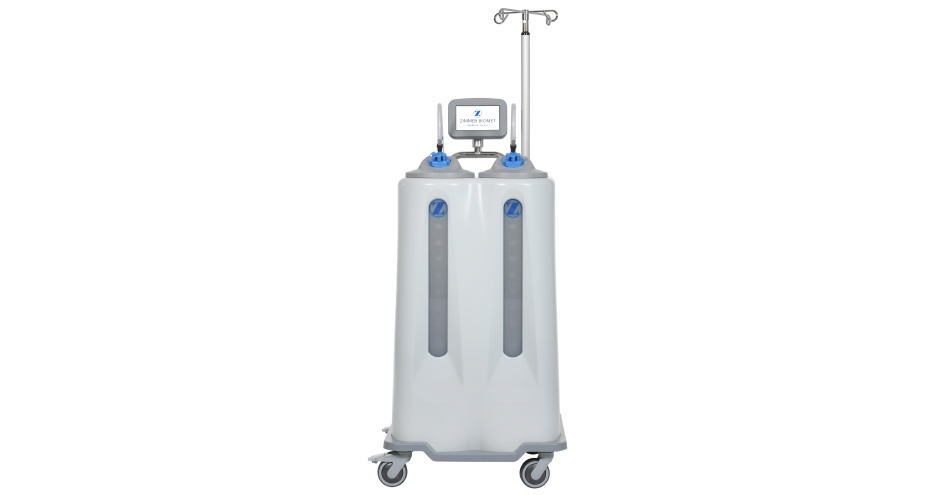
The IntelliCart System from Zimmer Biomet delivers advancements in day-to-day OR fluid and smoke waste management technology. With market leading 34-liter fluid capacity, extra quiet vacuum pump at only 47.5dB (3.87 sones), clog-free suction manifolds with integrated high-flow filters and portable smoke evacuation, the IntelliCart System makes managing surgical fluid and smoke waste hazards simple and convenient.