The delivery of a sterile, ready-to-use medical device is vital to delivering safe and effective patient care. Although conceptually a simple idea, the actual processes involved in delivering a sterile medical device to the sterile field involves a complex web of activities and stakeholders.
The theme of the 2019 Kilmer Conference, held in Dublin, Ireland, was “Collaborate to Innovate.” During the conference, a group of packaging-minded professionals, including health care delivery professionals, packaging engineers, sterility assurance professionals, medical device manufacturers, academicians and standards professionals held an ad hoc meeting. The opening question for participants was, “Are there any issues with sterile packaging that should be addressed collaboratively by the industry?” This open-ended question elicited a wide range of answers, all of which added up to the answer being “yes.”
The group’s most significant realization was that the chain of sterile packaging stakeholder groups exist within a series of silos. As a result, the group’s mission became breaking down those silos and working together. What started as an ad hoc meeting has evolved into a multidisciplinary effort to innovate the medical packaging space. Thus, the Kilmer innovations in Packaging (KiiP) project group was born.
The Last 100 Yards
 Each KiiP project group is responsible for defining its mission, scope, objectives and criteria for identifying and implementing projects. One of the groups, dubbed “The Last 100 Yards” is tasked with assessing sterile-packaged devices after they are delivered to the health care organization (including centralized distribution centers).
Each KiiP project group is responsible for defining its mission, scope, objectives and criteria for identifying and implementing projects. One of the groups, dubbed “The Last 100 Yards” is tasked with assessing sterile-packaged devices after they are delivered to the health care organization (including centralized distribution centers).
To that end, the group developed a survey targeting those within health care organizations responsible for handling sterile packaging. This included materials management, logistics, sterile processing professionals and staff involved in preparing and using the sterilized items for delivery of patient care. The survey focused not just on the items delivered sterile to the health care organization but also those prepared and sterilized by the health care organization itself. The objective of the survey was to identify areas for in-depth research and, ultimately, opportunities for improving the delivery of sterile items.
With 1,700 respondents answering some or all of the 61 questions in the survey, the level of participation was remarkable. Although sterile processing professionals were by far the largest group, a considerable number of operating room professionals also participated. A lesser number of respondents reported working in materials management/logistics and infection control.
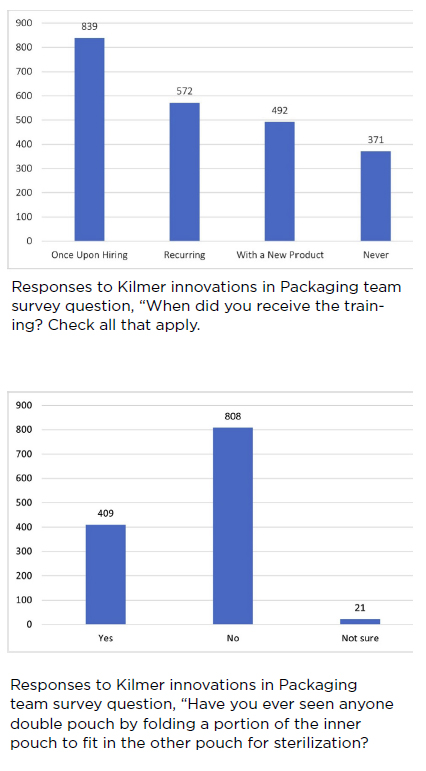
Informative insights were gained from the survey results, including those pertaining to training-related questions.
As with the survey among IAHCSMM members, the KiiP team survey focused on “actual practice” questions. During real-life events, nonideal practices can and do occur, and this survey focused on those nonideal practices. The survey took a different approach, asking respondents whether they “had ever seen a coworker do X. “The responses revealed the need for further education and collaboration between end users and industry.
The KiiP project group continues to review and analyze the survey results. It is anticipated that a number of follow-up studies, articles and white papers will be generated in the coming months. The KiiP team will share these with the industry as they become available. For further information on the KiiP movement and how to join the team, follow it on LinkedIn.
This is an excerpt from AAMI’s STERILIZATION CENTRAL column. Additional updates concerning KiiP team survey results can be found at aami.org/news.
Authors
- Ralph J. Basile, MBA, is vice president of marketing and regulatory affairs at Healthmark Industries in Fraser, MI.
Email: ralphjb@usa.net - Jennifer Benolken, CPPL, is MDM & regulatory specialist, packaging engineering, Tyvek, Medical Packaging, DuPont.
Email: jennifer.a.benolken@dupont.com - Malinda Elammari, ST, CSPM, CSPDT, CSIS, CFER, CRCST, CIS, CLSSGB, is interim director of education and quality at the Medical University of South Carolina in Charleston, SC.
Email: malinda.elammari@gmail.com - Erin Kyle, DNP, RN, CNOR, NEA-BC, is editor-in-chief of the Guidelines for Perioperative Practice at the Association of periOperative Registered Nurses in Denver, CO. Email: ekyle@aorn.org
- Jane Severin, PhD, CPPL, is vice president of technical solutions at Network Partners in Northville, MI.
Email: jane.severin@networkpartners.com - Katherine Olson, BS, is a staff packaging engineer at MicroAire Surgical Instruments in Charlottesville, VA.
Email: katherine.olson@microaire.com - Teri Meadow, MBA, is a healthcare market manager at American Packaging Corporation in Grand Rapids, MI.
Email: tmeadow@americanpackaging.com