Stryker Corp. has announced that more than 180 million SurgiCount Safety Sponges have been used in an estimated 10 million surgeries around the United States in the past five years.
Stryker Corp. has announced that more than 180 million SurgiCount Safety Sponges have been used in an estimated 10 million surgeries around the United States in the past five years.
“The successful implementation of SurgiCount at nearly 500 hospitals nationwide demonstrates the difference hospitals can make in strengthening patient-safety protocols,” said Dylan Crotty, vice president and general manager of Stryker Surgical. “SurgiCount can help protect a hospital’s patients, staff and bottom line by helping to reduce the risk of the most common surgical error, retained sponges.”
Despite efforts by hospitals nationwide to improve patient safety, retained surgical items (RSIs) continue to be the No. 1 reported surgical “never event.”
Numerous independent organizations – including The Joint Commission, the Association of periOperative Registered Nurses and the American College of Surgeons – recommend the use of adjunct technology to supplement manual sponge counting to reduce the risk of retained sponges.
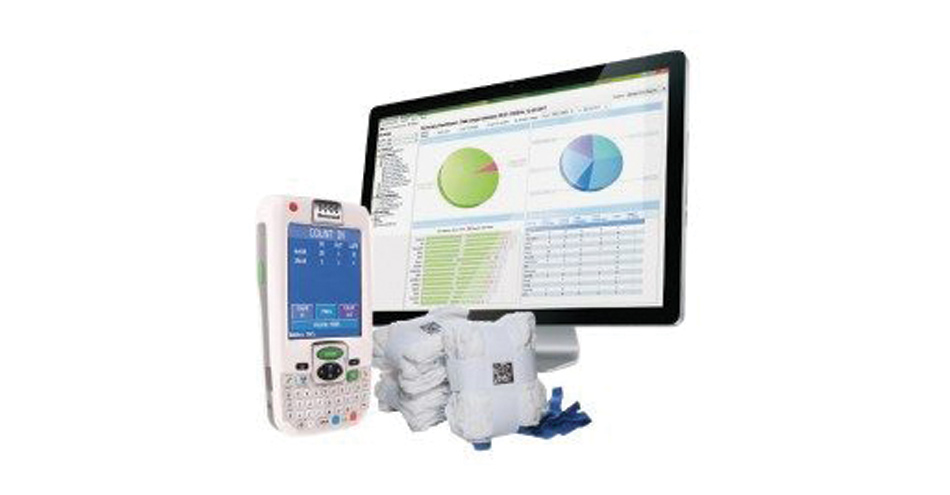
The SurgiCount Safety-Sponge System utilizes uniquely identified sponges and towels to provide a precise, real-time count so the surgical team can close a procedure – and a patient – with confidence. Unlike the traditional manual counting procedure, which relies on a whiteboard that is erased at the end of a procedure, a record of the SurgiCount-verified correct count is maintained in the hospital’s SurgiCount 360 software so that surgeons, nurses and hospital administrators have a permanent record of the verified count.
When used in conjunction with the manual counting process, SurgiCount significantly reduces the risk of retained sponges by addressing the problem of false-correct counts. The SurgiCount system is currently in use in more than 480 hospitals nationwide, and in an estimated 10 million-plus procedures, the system has never failed to identify a retained sponge.