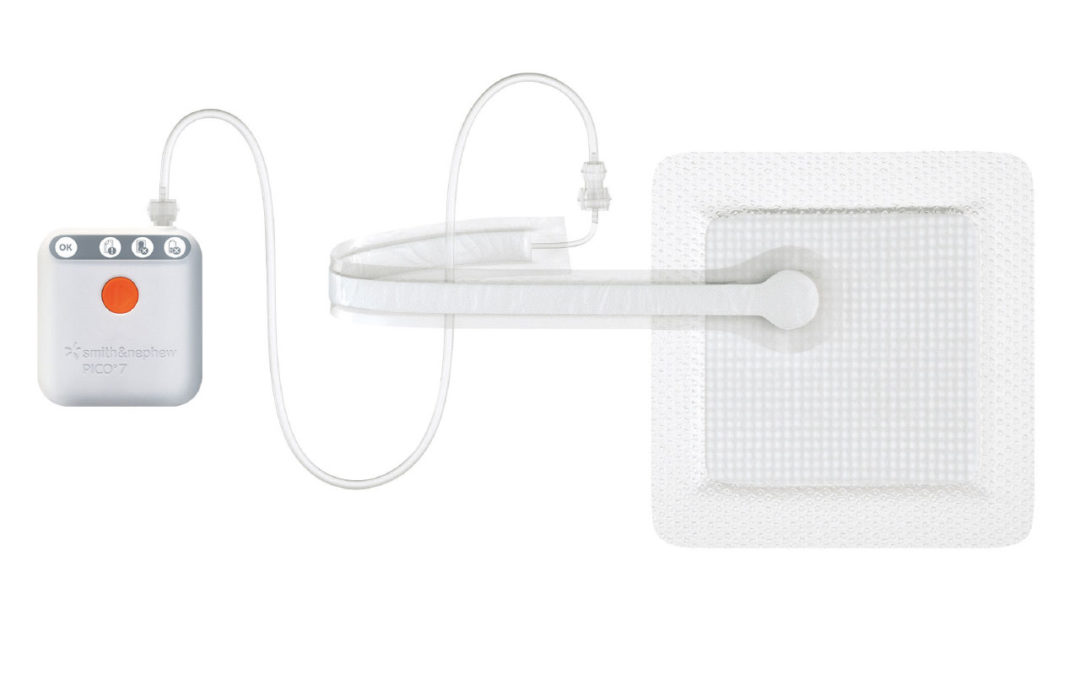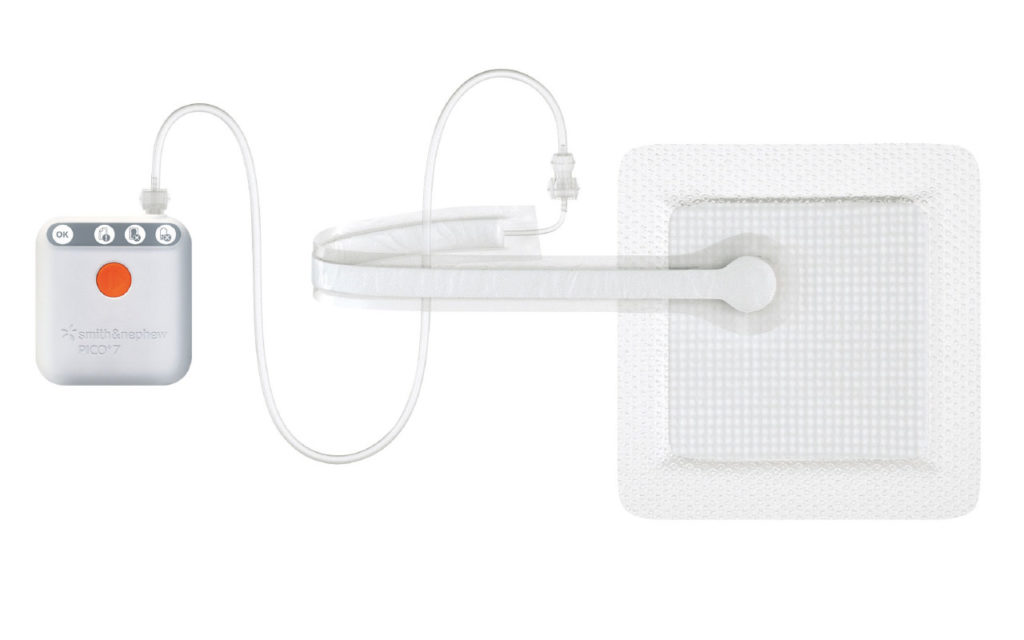
PICO Single Use Negative Pressure Wound Therapy is a negative pressure wound therapy system that raises the level of care:
- Indicated for use on closed surgical incisions and open wounds
- Manages low to moderate levels of exudate[1-3]
- Delivers compression-like therapy to the wound, wound margin and periwound[4]
- Canister-free and portable, which can help improve patient mobility and increase[5-6] satisfaction rates[7]
- Provides therapy for up to 14 days with PICO 14 and 7 days with PICO 7/7Y
- Waterproof dressing, allowing patients the ability to shower[5]
[1] Malmsjö M. et al. Biological effects of a disposable, canisterless Negative Pressure Wound Therapy system. Eplasty 2014; 14:e15. [2] Data on File DS/18/015/R. Summary Wound Model Report for Opal PICO 7. January 2018 [3] Data on file reference 1102010 – Bacterial Barrier Testing (wet-wet) of PICO dressing with a 7 day test duration against S.marcescens; Helen Lumb, February 2011. [4] Smith & Nephew January 2018. Outcomes following PICO compared to conventional dressings when used prophylactically on closed surgical incisions: systematic literature review and meta-analysis. Internal Report. EO/ AWM/PICO/004/v1. [5] Hurd, T., Trueman, P., & Rossington, A. Use of portable, single use negative pressure wound therapy device in home care patients with low to moderately exuding wounds: a case series. Ostomy Wound Management. Volume 60. Issue 3. March 2014. [6] WMP.11446.UEF/R3 Project Fairbanks Human Factors Summary Report Issue 5. G Walker, May 2017. [7] Kirsner R, Dove C, Reyzelman A, Vayser D, Jaimes H. A prospective, randomized, controlled clinical trial on the efficacy of a single-use negative pressure wound therapy system, compared to traditional negative pressure wound therapy in the treatment of chronic ulcers of the lower extremities. Wound Repair Regen. 2019 Sept;27(5):519-529.