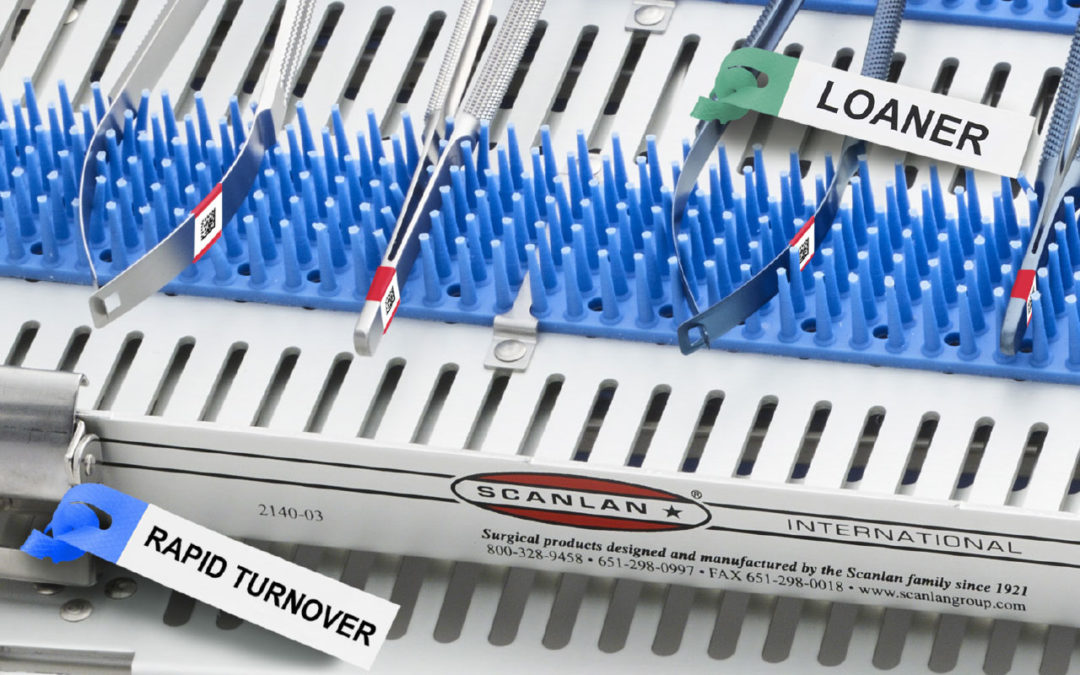
Scanlan Surg-I-Band Data Matrix Color Coding Barcode offers hospitals an excellent system to organize and track surgical instrumentation and devices. Combining the benefits of Surg-I-Band color coding with the technology of bar coding, Surg-I-Band Data Matrix provides a fast, easy and precise organizational system. The EASY-TAG Mini is designed to answer the need for a cost-effective, easy, secure method of tracking instruments, trays, equipment and repairs through cleaning, decontamination and sterilization processes.
For more information, visit www.scanlaninternational.com.