Medline has announced the release of its Transcatheter Aortic Valve Replacement (TAVR) drape, the latest addition to the company’s robust surgical drape product line that is designed to streamline operations and facilitate optimal patient care within the perioperative environment.
With over 300,000 TAVR procedures conducted since the procedure gained FDA approval in 2011, TAVR procedures seek to replace diseased aortic valves with a man-made valve by accessing the femoral artery with a catheter inserted through the groin. As an alternative to open-heart aortic valve replacement surgery, the replacement valve is guided through the femoral artery catheter into the heart where it is expanded upon reaching the diseased aortic valve.
“A TAVR procedure gives high-risk patients, that may not be able to go on cardiopulmonary bypass or even under general anesthesia, a life-saving option by replacing a diseased aortic valve with a man-made valve by accessing the femoral artery with a catheter inserted through the groin,” said Angela Pfeiffer, CST, CFA, perioperative clinical specialist at Medline. “As healthcare facilities are seeing an uptick in the volume of TAVR procedures that often require the use of specialized custom drapes, Medline created a solution to ensure cardiologists have what they need to provide the very best patient care.”
Conducted in over 715 hospitals, TAVR procedures are complex, advanced procedures requiring highly skilled physicians to insert a catheter into the femoral artery and run it all the way up into the heart to facilitate valve replacement. If an issue is uncovered during the procedure, there may be a need to quickly transition to an open-heart procedure, which could require additional equipment or preparation.
As a global leader in surgical drapes, Medline saw the need to ensure these highly skilled clinical teams are prepared no matter what they encounter in performing minimally invasive TAVR procedures. Medline’s new TAVR drape is the first of its kind and offers a flexible solution to sustain a minimally invasive procedure or if the need arises, immediately convert to a fully invasive open-heart procedure.
“TAVR procedures combine the skills and expertise of both cardiologists and cardiothoracic surgeons and Medline’s innovative sterile TAVR drape is designed to support the way these experts perform procedures in a combined solution,” said Angela Carranza, BA, CST perioperative clinical resources manager at Medline. “As a global leader in healthcare, it’s important for us to be innovative because we’re a solutions-driven partner committed to transforming healthcare, not just a product vendor.”
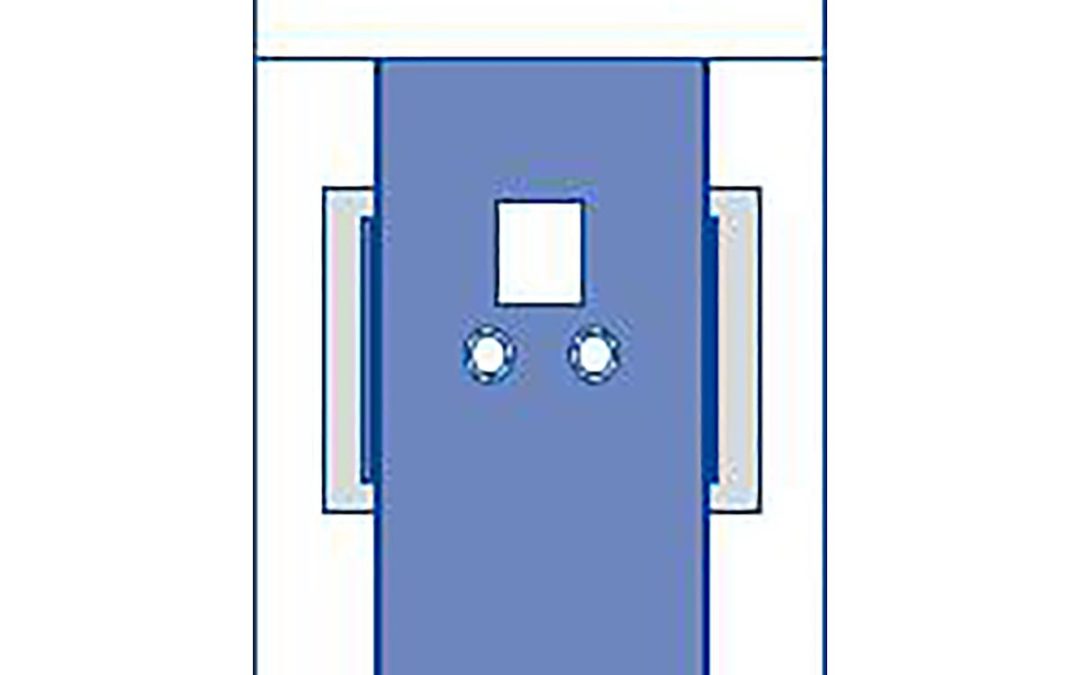
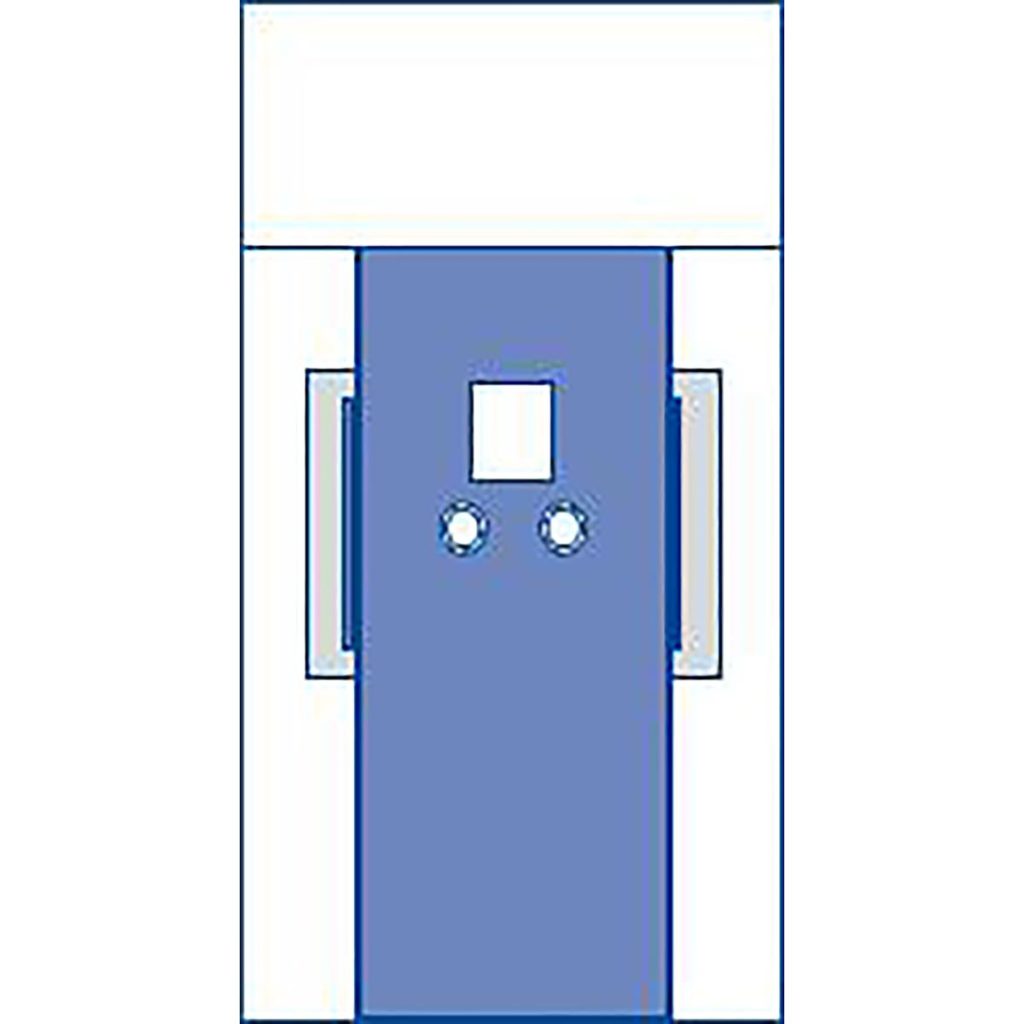
The newly released TAVR drape features two femoral access points for catheter insertion and a chest fenestration that enables a seamless, sterile transition to an open-heart procedure. The drape spans over 196” to cover various sizes of Cath Lab or hybrid operating room procedure tables while offering absorbent reinforced material surrounding all access points and dual liquid collection pouches for liquid control.
For more information, visit medline.com.