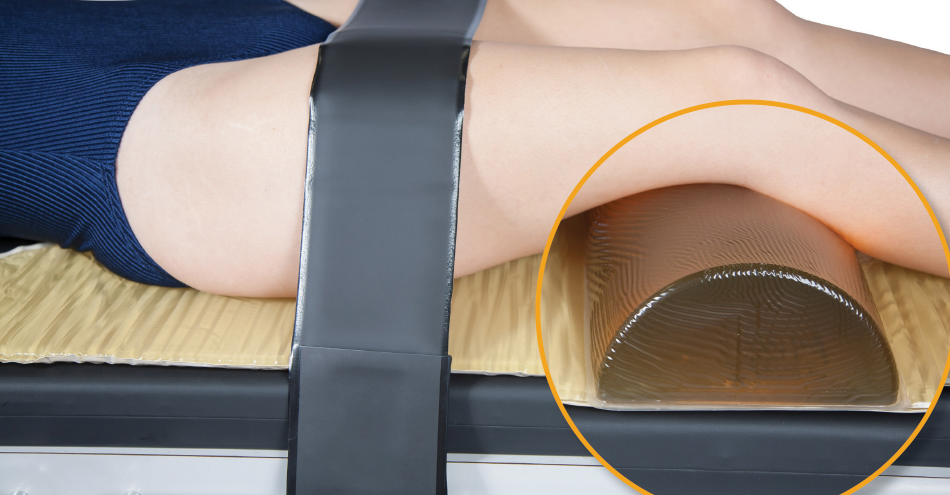
Patient positioning devices used under the knees should run the entire width of the surgical table pad. Most dome positioners are 14-inches long and are used under the knees during supine surgeries. In order for this positioner to cover the entire table many nurses put two positioners together. This solution hangs off of the edge of the table, may slide during surgery and cause pressure sores if skin falls between the cracks. The Action Dome Positioner (40603L) solves this problem, with 20-inches of Akton polymer gel to cover the table end to end. It redistributes the weight across the entire table.