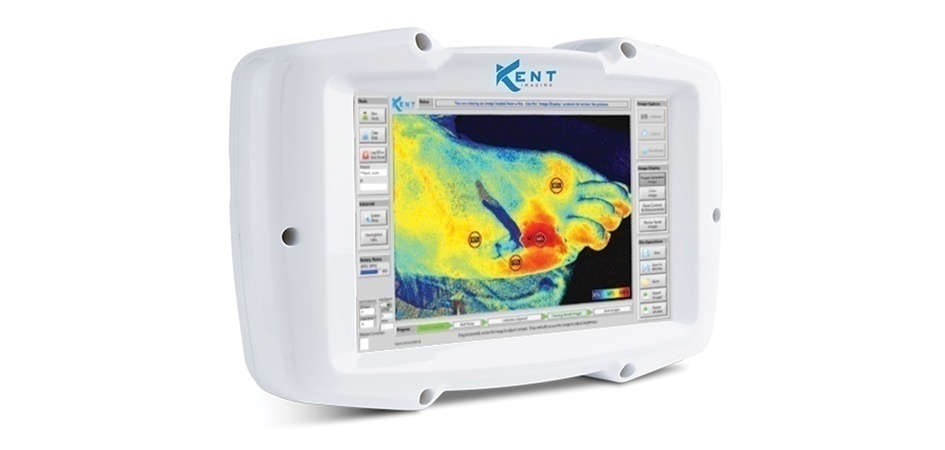
Kent Imaging, a provider of near infrared imaging solutions, has received CE Mark approval for the Kent multispectral medical imaging device (KD203). The device is also listed as a Class II medical device with the FDA and Health Canada. This allows Kent to expand its markets with distribution in Europe.
Kent Imaging has been working with European Research partners for the past 6 years to gather and publish clinical results using the Kent Device.
“Translational research is the word of the time,” said Dr. Flavio Coceani, Scuola Superiore Sant’Anna in Pisa, Italy. “Kent embodies this hallmark inasmuch as it moves an idea from the bench to the medical practice in a simple, cost-effective format.”
Kent Imaging’s device is the newest advancement in tissue oxygenation and perfusion imaging. The imaging device is handheld, cordless, portable, non-invasive and eliminates the need for tissue contact or injection of dyes. The Kent device operates with similar speed of a point and shoot camera and provides insight into the availability of oxygenated blood in tissue. This enables rapid and improved decision-making throughout the dynamic treatment path and helps to avoid unnecessary complications or corrections across the multiple points of care.
“We are very excited about obtaining CE Mark for our handheld device,” said Pierre Lemire, CEO of Kent Imaging. “This is another key milestone in Kent’s growth strategy. We can now engage with our European partners to bring our innovative technology to the European medical community.”
The Kent device has gained acceptance in both flap and implant-based reconstruction, abdominal wall surgery, colorectal surgery, cardiac use, vascular surgery and various types of wound care. The device is available for use in the operating room, clinics, and other care settings.