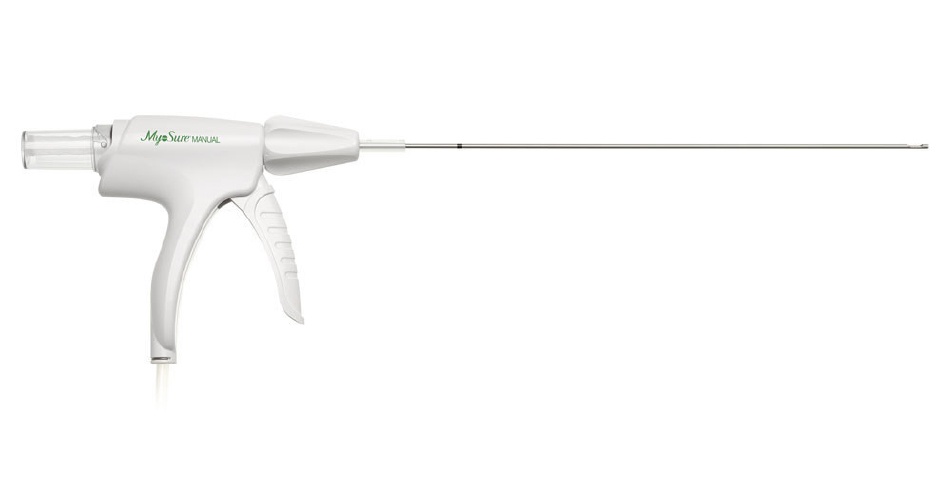
Hologic Inc. has announced that the MyoSure MANUAL device, which was designed to simplify tissue removal procedures regardless of setting, has received CE mark in Europe. The MyoSure MANUAL device joins the MyoSure suite of gynecologic surgical products that offer simple and effective solutions to resect and remove tissue.
Hologic offers the complete MyoSure system, which is a minimally invasive hysteroscopic treatment for women with problematic tissue that requires no cauterization, ultimately preserving uterine form and function. The system enables quick and convenient removal of tissue, including a range of fibroids and polyps that may be the root cause of abnormal uterine bleeding (AUB), which affects up to 30 percent of pre-menopausal women.
“There is an increased demand to simplify tissue removal procedures regardless of where they are being performed, in-office or in the theater. We continuously look to meet this need by developing innovative solutions that provide flexibility and convenience for both physicians and patients in any setting,” said Edward Evantash, M.D., medical director and vice president of medical affairs, Hologic. “The MyoSure MANUAL device is designed to simplify tissue removal procedures in theatre, ambulatory or outpatient settings, requiring minimal set up and no fluid management capital equipment, while offering direct visualization when used with the MyoSure hysteroscope.”
The MyoSure MANUAL device has a fully integrated vacuum with no external suction required. It involves the support of a one-liter saline bag – there is no need for a controller or additional fluid management capital equipment. The clear tissue trap allows visual confirmation of tissue removed during the procedure and holds up to 4g of tissue; it also detaches to send to pathology. In addition, the MyoSure MANUAL device gives physicians multi-function control of the 360-degree blade. Other devices in the MyoSure suite of products include the MyoSure, MyoSure REACH, MyoSure XL and MyoSure LITE devices.