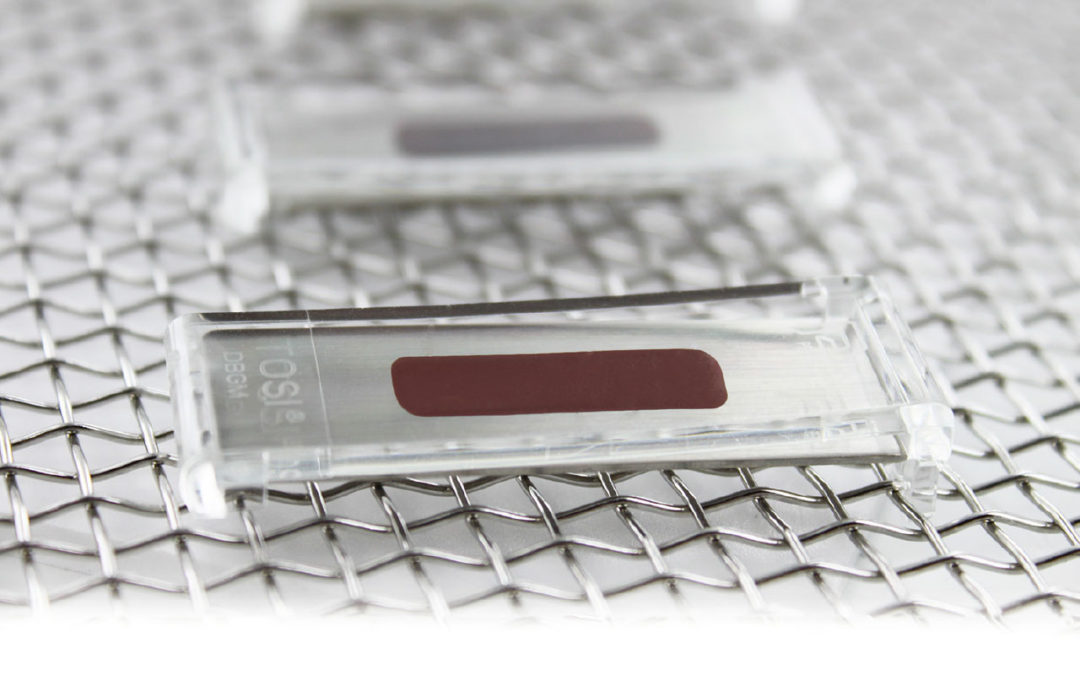
TOSI is the first device to provide a consistent, repeatable and reliable method for evaluating the cleaning effectiveness of the automated instrument washer. This is possible because the blood soil is manufactured to exacting specifications each and every time. When metered onto the stainless steel plate, the TOSI is completely analogous to a stainless steel instrument soiled with dried blood. Placed in the see-through plastic holder, the challenge is identical to the areas of instruments typically hidden from view (i.e., box locks). The routine use of this test will help insure that your instrument washer is performing at a consistent level, enhancing the routine visual inspection of instruments.
TOSI is the first device to provide a consistent, repeatable and reliable method for evaluating the cleaning effectiveness of the automated instrument washer. This is possible because the blood soil is manufactured to exacting specifications each and every time. When metered onto the stainless steel plate, the TOSI is completely analogous to a stainless steel instrument soiled with dried blood. Placed in the see-through plastic holder, the challenge is identical to the areas of instruments typically hidden from view (i.e., box locks). The routine use of this test will help insure that your instrument washer is performing at a consistent level, enhancing the routine visual inspection of instruments.
For more information, visit www.hmark.com.