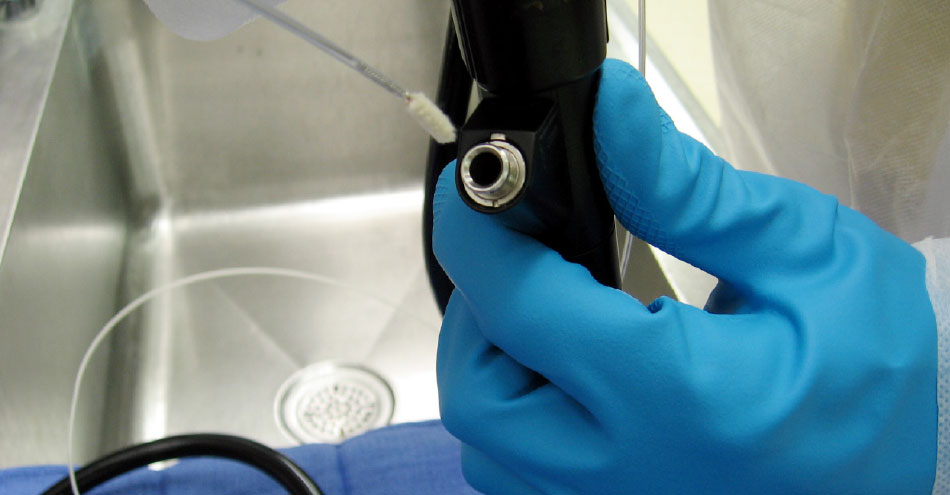
Test the cleanliness of the biopsy channel of the flexible endoscope. The EndoCheck is a miniature chemistry kit that is simple to use and interpret. Simply swab the biopsy channel of the scope with the included soft-tipped long probe, clip off the swab into the vial. Mix the activating agent, shake vigorously, wait and then check for a color change. Depending on the type of test used, a color change indicates that blood residue or protein residue remains in the channel, and should be reprocessed. EndoCheck complies with ASTM Guide D7225.








