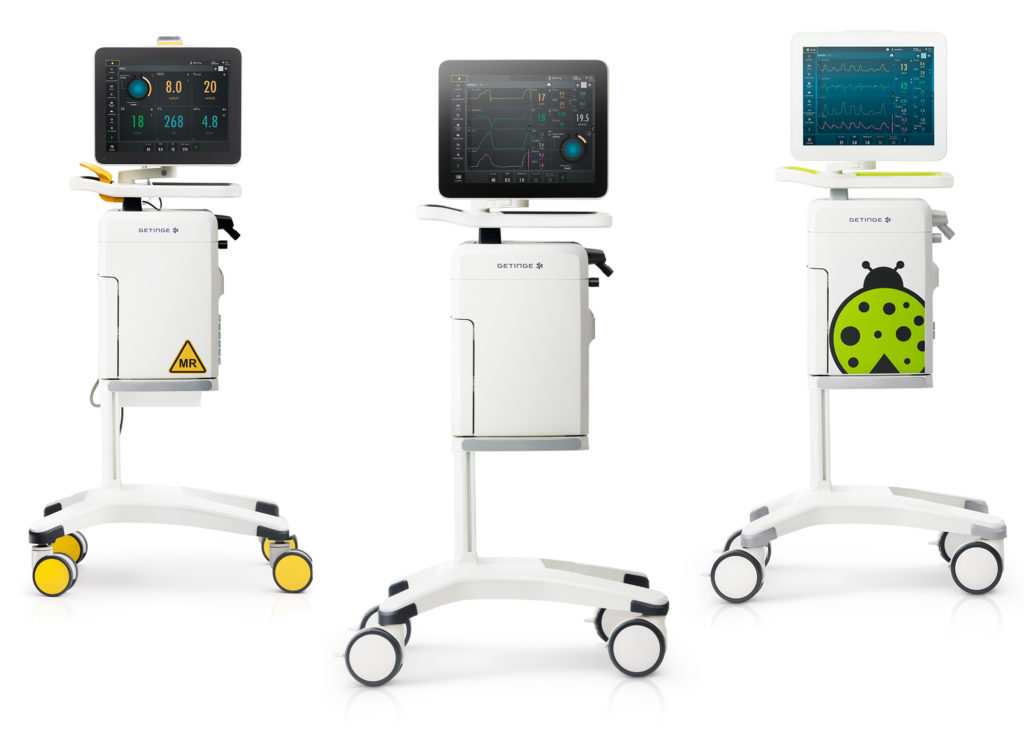
Getinge announced clearance from the U.S. FDA of several new software options for the Servo-u and Servo-n ventilators. In addition to the latest software upgrades, Getinge also received clearance for the new Servo-u MR ventilator for the MRI room.
“The COVID-19 pandemic and the heightened awareness of respiratory health has driven the need for personalized ventilation solutions for critically ill patients. Now more than ever, options for personalized lung protection and personalized weaning solutions are at the forefront of respiratory patient health. Getinge strives to support clinicians and patients by optimizing lung protection and delivering solutions for personalized ventilation,” said Eric Honroth, president, Getinge North America.
With this software upgrade for the Servo-u and Servo-n combined, Getinge adds several new functionalities and options across all patient categories – adult, pediatric and neonatal. Getinge broadens its portfolio of lung-protective tools, including Automatic Stepwise Recruitment maneuver (Auto SRM), a standardized and automated workflow that guides lung recruitment and helps clinicians identify a personalized PEEP that provides the lowest driving pressure, which is a variable strongly associated with patient survival in ARDS[1]. Stress index and Transpulmonary pressure monitoring, including key parameters for assessment of lung stress during controlled and spontaneous ventilation, complements the lung protective toolkit, which was designed to optimally divide the cognitive workload between the clinician and the ventilator.
Additionally, the clearance includes Heliox therapy. Heliox is a mixture of helium and oxygen that facilitates laminar flow and minimizes airway pressure due to its low density. This helps reduce the work of breathing (WoB) of patients suffering from obstructive lung diseases.
Getinge also received clearance to introduce the Servo-u MR to the U.S. market, a complement to the Servo Family, expanding Getinge’s platform of ventilators into the MRI room. Designed to guide the ventilator into a safe position, the Servo-u MR includes a magnetic field indicator with visual and audible alerts and an auto-lock handle that locks all four wheels as soon as the clinician releases the ventilator.
“We are seeing a transformation in the way healthcare providers view respiratory health,” said Honroth. “With this clearance, we are excited to be part of driving this transformation, working hand in hand with experts and clinicians.”
Getinge remains committed to innovation in ventilation platforms. With its rich legacy of firsts, Getinge is proud to bring innovative products and solutions to clinicians through this most recent 510k clearance.
The new options and the Servo-u MR ventilator are expected to be available in the U.S. in July 2021.
Read more about Getinge’s Servo ventilators.
1. Amato M, Meade M, Slutsky A, et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N Engl J Med. 2015; 372:747–755.










