COMMENCE Trial Four-Year Data Presented at the AATS 99th Annual Meeting
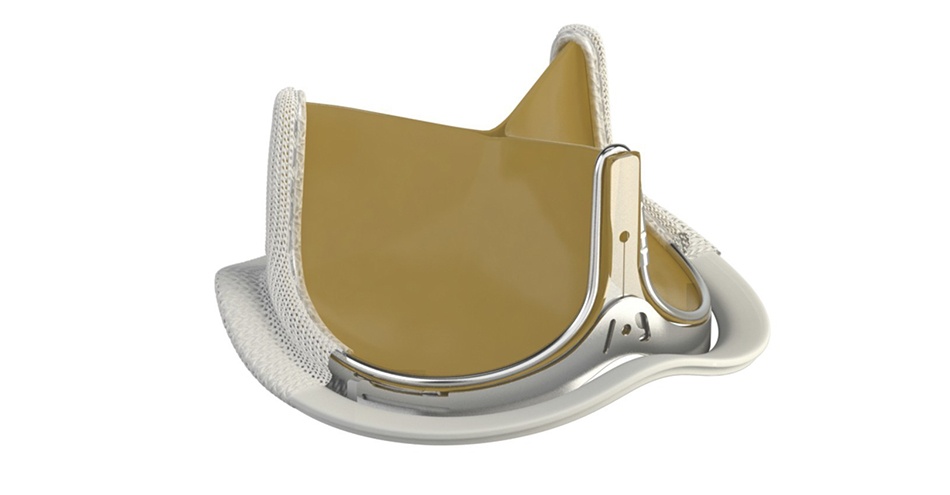
INSPIRIS RESILIA aortic valve
Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced results from the COMMENCE clinical study. The data show that Edwards’ bioprosthetic surgical aortic valves featuring the company’s novel RESILIA tissue platform continued to demonstrate favorable safety and hemodynamic performance through a median of four years follow-up, with no events of structural valve deterioration (SVD). The COMMENCE study enrolled 694 patients, 144 (21 percent) of which were under the age of 60 when they had surgical valve replacement. The data were presented at the American Association for Thoracic Surgery’s (AATS) 99th annual meeting.
“Patients undergoing valve replacement are living long lives and remaining more active through their later years, increasing the need for advanced valve replacement technologies,” said John D. Puskas, MD, principal investigator for the COMMENCE study. “The absence of structural valve deterioration in these patients is extremely encouraging and highlights the potential of valves containing RESILIA tissue for patients who may otherwise opt for a mechanical valve, which requires long-term use of blood thinning medications.”
Edwards’ innovative and proprietary RESILIA bovine pericardial tissue incorporates a novel integrity-preservation technology that may eliminate a key factor in calcification leading to valve deterioration. The technology also allows the valve to be stored under dry packaging conditions, facilitating ease of use in the operating room.Key findings from the study include:
- There were no cases of early or late structural valve deterioration or valve thrombosis.
- Mean transvalvular gradients at 1, 2, 3, and 4 years of follow-up were 10.2±4.6, 10.2±4.5, 10.8±6.0, 11.0±5.6 mm Hg, respectively.
“Edwards takes pride in our ability to innovate new technologies, such as RESILIA tissue, that can help improve long-term patient care and outcomes,” said Daveen Chopra, Edwards’ corporate vice president, surgical structural heart. “The RESILIA tissue platform was created with a vision to extend valve durability for a more active patient population, while reducing the need for long-term use of blood-thinning medication. These encouraging COMMENCE trial data suggest that we are on the path toward realizing that vision.”
The COMMENCE study is a prospective, non-randomized, multicenter, single-arm investigational device exemption (IDE) trial at 27 clinical sites across the United States and Europe. The trial enrolled 694 patients age 18 and older with diagnosed aortic valve disease and scheduled to undergo aortic valve replacement with or without coronary artery bypass graft (CABG). The COMMENCE trial is a PMA-approved study designed to evaluate the safety and effectiveness of surgical aortic heart valves with RESILIA tissue in this patient population for up to five years, with a subset of patients being evaluated through 10 years. At this stage, the study has recorded 2,533 patient-years of follow-up.
Additional five-year data from a European feasibility study of a bioprosthetic aortic valve with RESILIA tissue were presented last month at the Heart Valve Society Annual Meeting. This study, a prospective, single-arm observational clinical trial of 133 patients conducted at two clinical sites in Europe, also yielded positive results. There were no events of structural valve deterioration throughout the study period.
Edwards will further evaluate the novel RESILIA tissue platform through the RESILIENCE trial, a longer-term study that will follow up to 250 patients under the age of 65 who received an Edwards valve with RESILIA tissue. The study will examine calcification levels, hemodynamic deterioration and valve failure at years 5, 7, 9 and 11 as early potential predictors of valve durability.
Dr. Puskas is a consultant to Edwards Lifesciences.