Cummins has reached an agreement with 3M to manufacture high efficiency particulate filters for use in 3M’s Powered Air Purifying Respirators, or PAPRs.
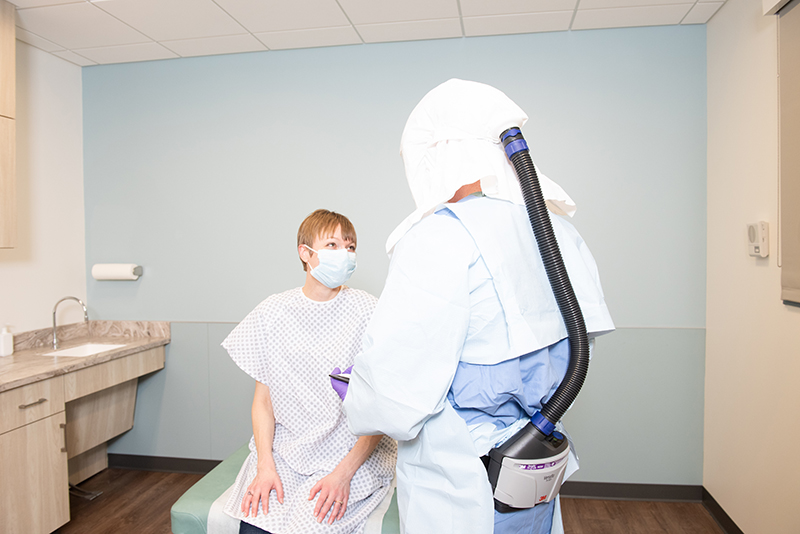
Pictured: A 3M Powered air purifying respirators (PAPR) in use.
The partnership has the potential to more than double the current production of filters for 3M’s PAPRs. The additional filters are needed as 3M has ramped up production of PAPRs to meet a surge in demand for personal protective equipment due to the COVID-19 outbreak.Powered air purifying respirators (PAPRs) are an important piece of equipment for front-line healthcare workers responding to the COVID-19 pandemic. PAPRs use a battery-powered blower that sends filtered air into a hood or head top that covers the wearer’s head or face; and can provide increased levels of respiratory protection, especially for critical healthcare situations such as aerosol generating procedures. PAPRs may also be more comfortable to wear for those who need respiratory protection for long periods of time.
“Cummins has been striving to find ways to help during the COVID-19 crisis,” said Tom Linebarger, Chairman and Chief Executive Officer of Cummins. “Working with 3M, we discovered our technologies and manufacturing expertise could be relevant as we partner in new ways to help protect healthcare professionals.”
Cummins will use existing manpower and equipment at its Neillsville, Wisconsin facility to pleat the media, assemble it into cartridge housings and do final testing before shipping the filters to Valley, Nebraska, where 3M’s PAPRs are manufactured. Production of the filters at Cummins’ Neillsville location is expected to begin by the end of April.
“3M continues to work around the clock to get personal protective equipment, including PAPRs, to the heroic healthcare workers and first responders on the frontlines of the COVID-19 fight,” said Mike Roman, 3M Chairman of the Board and Chief Executive Officer. “Our partnership with Cummins will help us produce more of this critical equipment in the coming months.”
Cummins’ Filtration business designs, manufactures and sells air, fuel, hydraulic and lube filtration, as well as chemical technology products for diesel and gas-powered equipment around the world.








