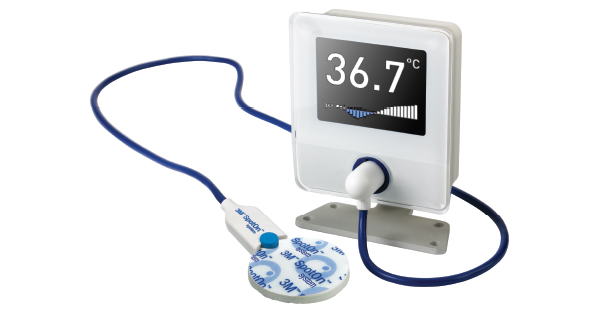
The 3M™ SpotOn™ system is a non-invasive, accurate core temperature monitoring system that continuously measures patient temperature with an affordable single-use sensor. Designed by the creators of 3M™ Bair Hugger™ therapy, the SpotOn system delivers temperature monitoring accuracy normally associated with more invasive systems like esophageal, bladder, rectal or PA catheters. In addition, the SpotOn system provides clinicians with a single temperature monitoring method that can be used through each phase of the perioperative journey, improving clinical efficiency while improving consistency, reducing opportunity for error and eliminating the duplication of effort required to purchase and carry multiple products. For more information, visit www.spotontemperature.com.