Teleflex Inc. has announced that its Arrow VPS Rhythm Device with Optional TipTracker Technology has been issued 510(k) Clearance to commercialize the device in the United States.
Teleflex Inc. has announced that its Arrow VPS Rhythm Device with Optional TipTracker Technology has been issued 510(k) Clearance to commercialize the device in the United States.
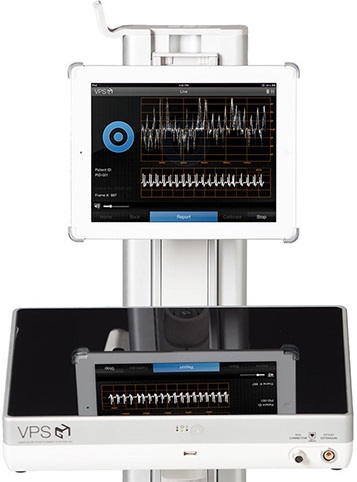
The Arrow VPS Rhythm Device is a simple and versatile solution that provides ECG-based tip confirmation in a highly portable and lightweight design.
The Arrow VPS Rhythm Device assists in placement and confirmation of a catheter tip in the SVC-CAJ (superior vena-cava-cavoatrial junction); it may be used with a broad range of catheter types and brands. Intravascular P-wave changes are saved as the catheter approaches the SVC, helping to identify the lower 1/3 of the SVC, near the CAJ, eliminating the need for confirmatory chest X-ray or fluoroscopy in adult patients. When paired with the single-use TipTracker Stylet for insertion of peripherally-inserted central catheters, the Arrow VPS Rhythm Device provides real-time visual navigation by tracing the catheter pathway with a blue line on a color screen. The Device has an expansive sphere of visual navigation to provide easy navigation of the PICC during insertion.
“This clearance positions us to provide catheter tip placement solutions that meet customer’s unique therapeutic and budgetary needs,” said Jay White, president and general manager, Vascular Division of Teleflex. “The VPS Rhythm Device with Optional TipTracker Technology will complement our existing Arrow VPS G4 Device, diversifying and strengthening our catheter tip positioning portfolio globally. Most importantly, it furthers our goal of making products that simplify clinician’s work and improve a patient’s care.”