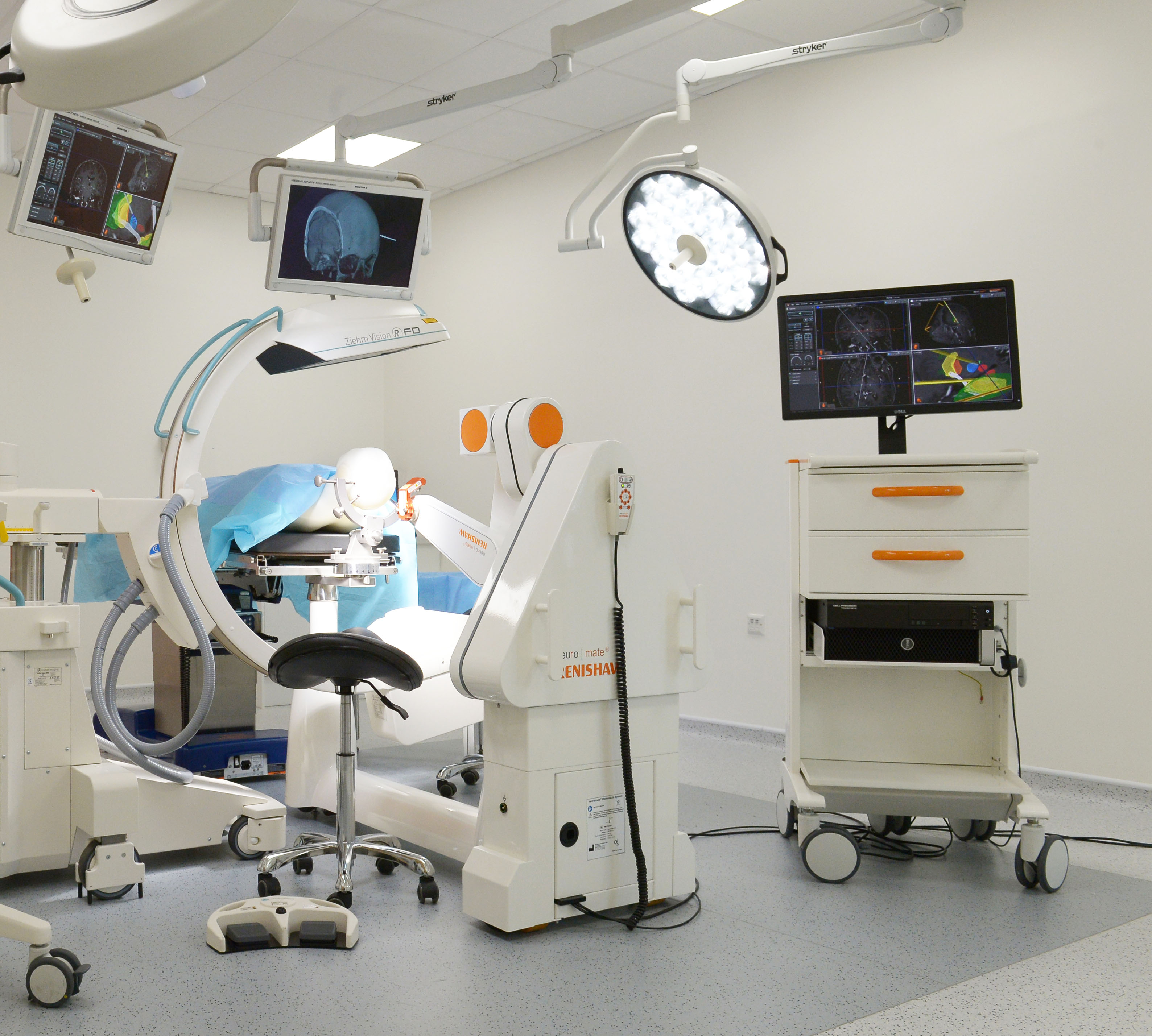
The Food and Drug Administration (FDA) has cleared the use of Renishaw’s neuromate® Gen III surgical robot with the neuroinspire™ surgical planning software in the United States. Both were previously cleared for use separately, but not in combination. This latest clearance means that neurosurgeons across America will be able to deliver surgical plans created using neuroinspire software directly using the neuromate surgical robot, helping to improve patient outcomes.
Renishaw’s neuromate robotic system for stereotactic neurosurgery provides a platform solution for several functional neurosurgical procedures. The neuromate robot has been used in thousands of procedures such as deep brain stimulation (DBS), stereoelectroencephalography (SEEG), biopsy and more.
The neuroinspire software assists with the planning of stereotactic procedures. Using 3D patient scan data, neuroinspiresoftware allows neurosurgeons to clearly visualise the safest surgical route to target. The software also allows neurosurgeons to visualise an image of implantable instruments in position along with a customisable safety zone.
Before obtaining this latest clearance, plans generated using neuroinspire software could be manually transferred onto a traditional stereotactic frame. With this latest clearance, customers can now export surgical plans from neuroinspiresoftware directly to the neuromate robot for efficient procedure execution.
“Hospitals in the UK and the rest of Europe are already using the neuromate surgical robot in combination with neuroinspiresoftware,” explained Andrew Dissington, Senior Project Manager at Renishaw. “Now, patients across the U.S. will be able to benefit from improved neuromate robot procedures for Parkinson’s, epilepsy and brain tumours.”
“SEEG procedures for epilepsy can involve 20 electrodes being implanted into the brain,” added Dissington. “Robotic surgery is much quicker to deliver than manually using a frame. The approval means that existing neuromate surgical robot users are able to take advantage of Renishaw’s intuitive, user-friendly software package.”
Renishaw offers a complete package from planning to delivery, which includes the supply of consumables and training. The company regularly updates its software offering, which can also be customised to meet specific needs using add-on modules.
For more information on Renishaw’s products for stereotactic neurosurgery, visit www.renishaw.com/neuro.