KARL STORZ Endoscopy-America Inc.’s system for performing Blue Light Cystoscopy with Cysview (BLCC) has received an Innovative Technology designation from Vizient Inc., the largest member-driven health care performance improvement company in the country.
The designation was based on reviews of BLCC by hospital experts who attended Vizient’s Innovative Technology Exchange during September 2016. The event provided medical technology suppliers the opportunity to demonstrate their product and gain direct feedback from 1,300 onsite clinical experts and health care providers on the impact their products may have on improving clinical care, safety, or benefits to an organization’s care and business model.
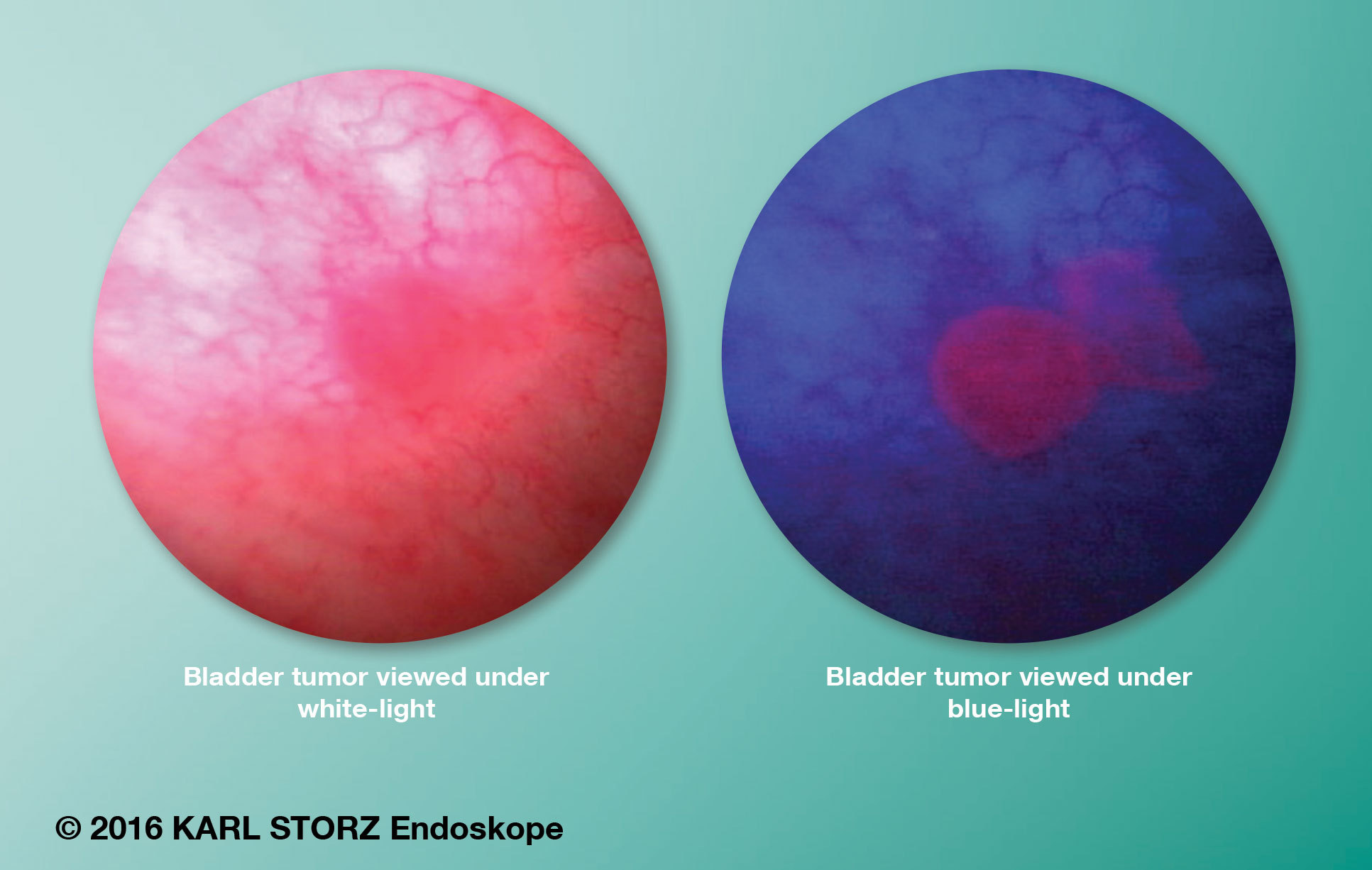
The BLCC system uses the KARL STORZ D-Light C Photodynamic Diagnostic (PDD) system in conjunction with Cysview, an optical imaging agent distributed in the U.S. by Photocure, Inc. The combined system is used to enhance the detection and management of Non-Muscle Invasive Bladder Cancer (NMIBC) in patients with known or suspected bladder cancer, based on prior cystoscopy. Use of the technology begins with placement of Cysview into the bladder prior to the procedure, where it accumulates in tumor cells and is converted into porphyrins within the tumor. When viewed with the D-Light C PDD system, the tumor cells appear red or pink in contrast with normal cells, which appear blue in color. The system is capable of delivering both conventional white light to illuminate the bladder during routine cystoscopy, as well as a special blue light to induce and view fluorescence after the placement of Cysview.
“Use of the KARL STORZ D-Light C PDD system for BLCC procedures gives vital new capabilities to physicians,” says John Martineau, Director of Marketing Urology, KARL STORZ. “Used as an adjunct to white-light cystoscopy, BLCC is the only FDA-approved technology that is shown to improve detection of bladder cancer tumors hence leading to improved and more comprehensive tumor resection.”
KARL STORZ Endoscopy-America, Inc., was previously recognized by Vizient, Inc. and also awarded the Innovative Technology designation in 2015 for its IMAGE1 Visualization Enhancement System.
Vizient, Inc. represents the combined strengths of the organizations formerly known as VHA Inc., University HealthSystem Consortium, Novation and MedAssets’ Spend and Clinical Resource Management.