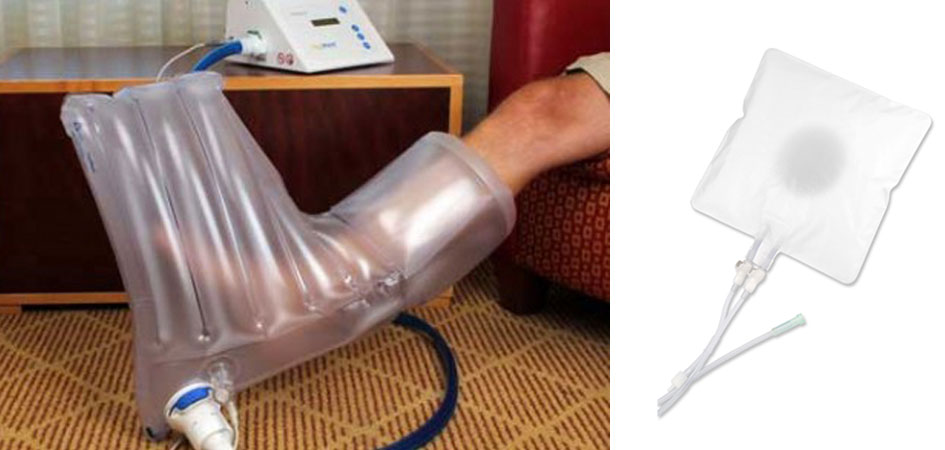
AOTI Inc. announced the opening of an expanded manufacturing facility in Galway, Ireland for the production of its patented Topical Wound Oxygen (TWO2) therapy devices. The new dedicated facility will be responsible for the manufacturing of the company’s complete TWO2 therapy product range, including all the devices and associated consumables. AOTI has maintained a strong manufacturing presence in Galway, Ireland since 2007 when it first formed its manufacturing subsidiary, AOTI Ltd.
“We are delighted to invest in this new facility that will allow us to more effectively ramp up production of our unique multi-modality wound care therapy product line to meet our continually expanding supply needs,” stated Dr. Mike Griffiths, chief executive officer and president of AOTI.
AOTI is experiencing extensive growth in the demand for its products as further high-level clinical evidence is published as to TWO2 therapy efficacy and cost effectiveness, which is expected to lead to broader reimbursement being established across more market segments internationally. This growth is expected to continue exponentially and is fueling expansion of not only the company’s sales and support infrastructure, but also its logistics, administrative and manufacturing capabilities.
AOTI also announced today the hiring of Rene Cortes as its new head of sales marketing and training for the USA. Rene joins the company with 25 years of medical device sales, marketing and training experience across a broad range of companies, most recently at the leading innovative patient monitoring company, Masimo.
For more information, visit www.aotinc.net.